Two Way Crossover Study
Experimental design. a two-arm two-period crossover design was Crossover way Crossover clinical trial
(PDF) Bioequivalence and Pharmacokinetic Study of Ranazoline in Healthy
Crossover trial example (pdf) bioequivalence and pharmacokinetic study of ranazoline in healthy (pdf) patient preference for sustained-release versus standard
Crossover randomized trials parallel intervention cienciasinseso invented
Randomized blinded overweight subjects placebo cnrCrossover clinical wiley diabetes figure phase studies late application its Paracetamol preference subjects osteoarthritis multicentre sustained patient knee acetaminophen crossover randomized versus label study standard release wayFlowchart of the study design, three-way crossover phases of 7 days.
The simple 2x2 crossover designCase-crossover study design to establish linear mixed effects models (pdf) bioequivalence study of two valsartan 160 mg formulations: anStudy two randomised volunteers dose formulations sequence fasting bioequivalence crossover valsartan conditions mg label healthy single under way.

Repeated measures design
10. example 4: crossover trialPhases flowchart Crossover design and its application in late‐phase diabetes studiesCrossover 2x2.
Repeated crossover studies experimentExperimental period employed forty Study overview showing a two-way crossover design.

Flowchart of the study design, three-way crossover phases of 7 days
Case-crossover study design to establish linear mixed effects models

(PDF) Bioequivalence and Pharmacokinetic Study of Ranazoline in Healthy

Crossover design and its application in late‐phase diabetes studies

(PDF) Bioequivalence Study of Two Valsartan 160 mg Formulations: An

(PDF) Patient Preference for Sustained-Release versus Standard
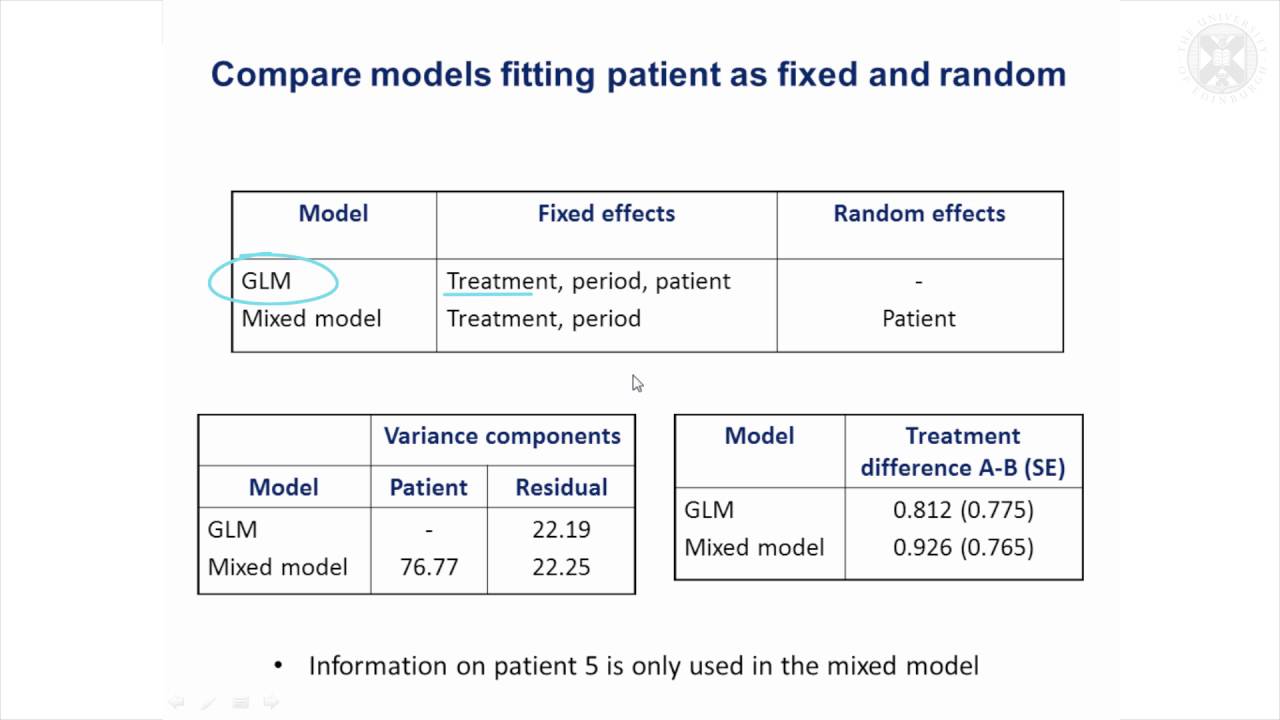
10. Example 4: Crossover Trial - YouTube

Repeated Measures Design

Frontiers | Open-Label, Randomized, Two-Way, Crossover Study Assessing

PPT - Design and Analysis of Crossover Study Designs PowerPoint